Are you a new member? Sign up now
Login area
| Sign up
Pharmacovigilance System in Lebanon
Pharmacovigilance System in Lebanon
One of the core functions of the Lebanese Ministry of Public Health (MoPH) is to safeguard the Quality, Safety and Efficacy of medical products (medicines, vaccines, biological and medical devices) at the national level. This task is carried out by the Quality Assurance of Pharmaceutical Products (QAPP) Program at MoPH, directed by Dr. Rita Karam which aims, in specific, to reinforce the implementation of quality standards relating to the safety of pharmaceutical products and to ensure that drugs reach the patient in a safe, effective and acceptable manner. The enhancement of patient safety is achieved by the project on the implementation of a National Pharmacovigilance (PV) System. Within the framework of the above mentioned project, the MoPH QAPP program developed a national system for spontaneous reporting of adverse events, and designated the PV Center at the Faculty of Pharmacy at the Lebanese University (LU). http://phcvg-lebanon.com/index.php/en/phcvg-n/I- Pharmacovigilance Definition
PV is defined by the WHO as the science and activities relating to the detection, assessment, understanding and prevention of Adverse Events (AEs) or any other medicine related problem. Its aims are to enhance patient care and patient safety and to support public health programmes by providing reliable, balanced information for the effective assessment of the benefit-risk profile of medicines and vaccines. PV addresses AEs of medicines, medicine errors, counterfeit/substandard medicines, lack of efficacy, abuse and misuse of medicines, and interaction of medicines.
II- Importance of Pharmacovigilance
Medicines and vaccines have changed the way in which diseases are prevented and treated. In spite of their benefits, medical products have unexpected effects. Some of these effects are unfavorable, ranging in severity, seriousness and frequency within the intended population. While medicines and vaccines are studied in well controlled clinical trials and reviewed by regulatory authorities with the aim of ensuring benefits outweigh risks, some adverse effects (AEs) are observed only once the product is authorized by regulators and used by a larger population in ‘real world conditions’, including special populations such as children, pregnant women and elderly. It is therefore critical that medical products continue to be monitored for their effectiveness and safety post release. In practice this means having in place a well-functioning PV system.
Improper monitoring of medicines and vaccines can lead to catastrophic consequences. In some countries, the AEs are ranked 4th to 6th on the mortality scale. The total percentage of hospital admissions due to such events is an average between 10-20%. Some healthcare systems spend around 15-20% of their budget on medicine-related AEs which adds up to a high economic expenditure. In response to these figures, every country must strictly adhere to the application of PV.
What are the Advantages of Pharmacovigilance System? It:
- Reduces medicine/vaccine-related problems leading to better treatment outcome
- Improves the quality of care offered to patients
- Improves patients confidence in professional practice
- Is a cost effective method of monitoring the safety of medicinal products throughout its lifetime
- Includes AEs reporting system which is the primary method of data collection used in most countries as it is an easy and fast way to submit an urgent health related issues
- Provides feedback information on medicine/vaccine-related problems reported nationally and internationally
III- About Pharmacovigilance
Pharmacovigilance System Mission
Detecting, assessing, understanding and preventing AEs
.
Pharmacovigilance System Vision
Paddling in the same direction of the PV international community to achieve safer use of medicines worldwide
Pharmacovigilance System Values

Stakeholders of the Pharmacovigilance System in Lebanon
The PV System in Lebanon is comprehensive and includes many stakeholders:
Pharmacovigilance System Mission
Detecting, assessing, understanding and preventing AEs
.
Pharmacovigilance System Vision
Paddling in the same direction of the PV international community to achieve safer use of medicines worldwide
Pharmacovigilance System Values

Stakeholders of the Pharmacovigilance System in Lebanon
The PV System in Lebanon is comprehensive and includes many stakeholders:
- The government is responsible for providing all the support needed for the national PV System through well-established national policy and action plan.
- The QAPPP at the MoPH, that is responsible for the implementation of quality standards related to the safety of pharmaceutical products, aimed at ensuring that medicines reach the patient in a safe, effective and acceptable manner. The QAPPP oversees the implementation of the PV System.
- The Lebanese National Pharmacovigilance Center ( LNPVC) at the Faculty of Pharmacy - Lebanese University : http://phcvg-lebanon.com/index.php/en/phcvg-n/
- The WHO-PIDM which is the forum where member states can collaborate in PV. The PIDM is responsible for policy issues, while the other partner, the Uppsala Monitoring Center (UMC) conducts operations.
- Other parties (e.g., Marketing Authorization Holder, Health-Care Providers, Public Health Programs, Expanded Program for Immunization (EPI) and Primary Health Care Centers and patients/consumers) responsible for reporting AEs which collaborate as main stakeholder to the PV System through submitting Individual Case Study Reports (ICSRs) to the LNPVC.
IV- Steps for the Implementation of Pharmacovigilance System in Lebanon: Regulations
Because of the seriousness of the situation, the MoPH decided to integrate strategies and regulations for medicines safety monitoring which included implementing the National PV System and the National Policy on the Safe and Rational Use of medicines and vaccines.
In this regard, the following chronological regulatory framework is considered to be the core building blocks related to the implementation of a PV system in Lebanon:
Because of the seriousness of the situation, the MoPH decided to integrate strategies and regulations for medicines safety monitoring which included implementing the National PV System and the National Policy on the Safe and Rational Use of medicines and vaccines.
In this regard, the following chronological regulatory framework is considered to be the core building blocks related to the implementation of a PV system in Lebanon:
- Ministerial Decree No.13370 (2004)
A Ministerial Decree No.13370 issued in 10/09/2004 from the Ministry of Education stated the creation of the Center for AEs of Drugs’ Monitoring in the Faculty of Pharmacy at the Lebanese University.
Ministerial Decree No.13370 (2004)
Ministerial Decree No.13370 (2004)
- Ministerial Resolution No. 1636 (2013)
The MoPH released several mandatory instruments including: Ministerial Resolution No. 1636 issued in 19/02/2013 to establish a committee at the QAPPP to examine AEs. Its responsibility covers the collection of AE-related data, review and evaluating this information, and communicating with the Center for AEs of Drugs’ Monitoring. Every AE collected and evaluated is then reported back to the Technical Committee (TC) of medicines at MoPH for decision making.
Minister's Decision No.1636 of 9/10/2013
Minister's Decision No.1636 of 9/10/2013
- Collaborative Agreement (2016)
A collaborative agreement between the Lebanese University and MoPH dated 24/02/2016 authorized the Center for Drugs Monitoring of Advers Events at the Faculty of Pharmacy of the Lebanese University to function officially as National Pharmacovigilance Center.
- A Strategic and Operational Plan (2016-2020)
A strategic and operational plan for a period of 5 years (2016-2020) was drafted for the MoPH in 2016. The main goals of the plan include upgrading the hospital accreditation and licensing systems as well as establishing Pharmacovigilance and post-marketing surveillance systems. Through the MoPH, QAPP applies for membership to the WHO-PIDM and Lebanon became an Associate Member in July 2018.
Strategic Plan for the Medium Term (2016 to 2020)
Strategic Plan for the Medium Term (2016 to 2020)
- PV Strategic Plan and Operational Plan (2020-2025)
A PV system strategic plan and an operational plan for the upcoming 5 years 2020-2025 was drafted and it details all the activities, objectives, regulations, departments, responsible personnel, partners, collaborators, timescale, and indicators.
- Ministerial Resolution No. 1438/1 (2019)
Related to work mechanisms for the PV project in Lebanon and assigns Dr. Rita Karam as PV coordinator between the MoPH, WHO and LNPVC
Minister's Decision No.1438 of 25/7/2019
- Ministerial Resolution No. 427/1 (2020)
The resolution is related to the Procedure for Reporting Adverse Drug Reactions Related to COVID-19 treatments.
Minister's Decision No.427 of 14/4/2020
Minister's Decision No.427 of 14/4/2020
- Ministerial Resolution No. 556/1 (2020)
The Regulation states the procedure for Reporting Adverse Drug Events related to the COVID-19 treatment by the responsible parties of Pharmaceutical Products and Drug Distributors
Minister's Decision No.556 of 28/5/2020
Minister's Decision No.556 of 28/5/2020
- Minister's Decision No.180/1 (2021)
Minister's Decision No.180/1 of 3/2/2021
PV Glossary of terms
Adverse Event Reporting Form for Medicines and Vaccines - English version
Adverse Event Reporting Form for Medicines and Vaccines- Arabic version
Instruction for filling the Reporting Forms- English & Arabic version
PV Glossary of terms
Adverse Event Reporting Form for Medicines and Vaccines - English version
Adverse Event Reporting Form for Medicines and Vaccines- Arabic version
Instruction for filling the Reporting Forms- English & Arabic version
- Minister's Decision No.181/1 (2021)
Clarifications regarding the Ministerial Decisions #180 & 181 released on 03/02/2021- 24/3/2021
PV Clarifications for MAHs on Ministerial Decisions#181- 22/2/2021
Clarifications regarding the Ministerial Decisions #180 & 181 released on 03/02/2021- 19/2/2021
- Memorandum No.8 (2021)
Minister's Memo No.8 of 8/2/2021
- Letter related to the Nomination of Hospital Pharmacovigilance Focal Points
V- Reporting of Adverse Events for Medicines and Vaccines
 Electronic Reporting: e reporting
Electronic Reporting: e reportinghttps://primaryreporting.who-umc.org/Reporting/Reporter?OrganizationID=LB
 Quality Assurance of Pharmaceutical Products Program: 01-830254 01-830255
Quality Assurance of Pharmaceutical Products Program: 01-830254 01-830255 Lebanese National Pharmacovigilance Center: 05-463652
Lebanese National Pharmacovigilance Center: 05-463652Pharmacovigilance Team
Dr. Rita Karam: Head of National Pharmacovigilance Program
Dr. Abeer Zeitoun: Senior Clinical and Technical Manager at the Pharmacovigilance Program
Dr. Myriam Watfa: Pharmacovigilance Program Consultant
Dr. Katia Iskandar, Pharmacovigilance Consultant
Dr. Sarah Rida El Sayed: National Pharmacovigilance Officer
Dr. Aya Ibrahim: National Pharmacovigilance Officer
For further information please visit the following link:
https://www.who.int/teams/regulation-prequalification/pharmacovigilance
Contact us:
Telephone: +961-1-830300 Ext: 254/5
Email: pv@moph.gov.lb
National Pharmacovigilance Safety Reports
Announcement January 15th, 2026:This section presents official national pharmacovigilance reports and signal analyses issued by the Lebanese National Pharmacovigilance Program, providing consolidated evidence on medications and vaccines safety data.
Adverse Events Following Medications and Vaccines Use in Lebanon
Lebanese National Pharmacovigilance Program Newsletter
- National Pharmacovigilance Program Newsletter - Issue 17 (January 2026)
- National Pharmacovigilance Program Newsletter - Issue 16 (October 2025)
- National Pharmacovigilance Program Newsletter - Issue 15 (July 2025)
- National Pharmacovigilance Program Newsletter-Issue 14 (April 2025)
- National Pharmacovigilance Program Newsletter-Issue 13 (January 2025)
- National Pharmacovigilance Program Newsletter-Issue 12 (October 2024)
- National Pharmacovigilance Program Newsletter-Issue 11 (July 2024)
- National Pharmacovigilance Program Newsletter-Issue 10 (April 2024)
- National Pharmacovigilance Program Newsletter-Issue 9 (January 2024)
- National Pharmacovigilance Program Newsletter-Issue 8 (October 2023)
- National Pharmacovigilance Program Newsletter-Issue 7 (June 2023)
- National Pharmacovigilance Program Newsletter-Issue 6 (April 2023)
- National Pharmacovigilance Program Newsletter-Issue 5 (January 2023)
- National Pharmacovigilance Program Newsletter-Issue 4 (October 2022)
- National Pharmacovigilance Program Newsletter-Issue 3 (June 2022)
- National Pharmacovigilance Program Newsletter-Issue 2 April 2022
- National Pharmacovigilance Program Newsletter-Issue 1 January 2022
Pharmacovigilance Activities
Pharmacovigilance Theory and Practice - USJ School of Pharmacy Educational Session Breifing - January 2026Training on the Lebanese Good Pharmacovigilance Practices (LGVP) – November 2025
%20%E2%80%93%20November%202025.jpg)
Second Oman Pharmacovigilance Symposium

Webinar Briefing- June 2022



Capacity Building- November 2021




Pharmacovigilance News

Briefing on the Official Launch of the Lebanese Good Pharmacovigilance Practices Guideline

1st Annual Lebanese Pharmacovigilance Conference- Briefing

Medicines Safety Takes Root in Lebanon

A 1-year analysis of adverse events following COVID-19 vaccination in Lebanon: a retrospective study
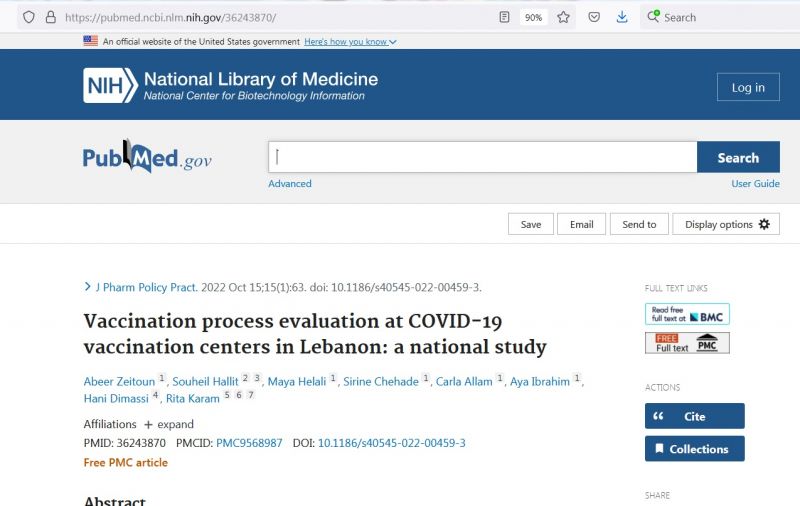
Vaccination Process Evaluation at COVID-19 Vaccination Centers in Lebanon: A National Study

Implementation of a PV System in Lebanon Review article

Launching Event- September 2022

ISQua Webinar Briefing- September 2022

TV Interview Briefing- September 2022
The Pharmacovigilance training sessions, organized by Dr. Rita Karam and Dr. Myriam Watfa took place four times between the time period of 26 February 2021 until the 19th of March 2021. The presentations were run by Dr. Karam, Dr. Katia Iskandar, Dr. Jihane Howayek, Dr. Hanine Abbas and Dr. Watfa and moderated by both Dr. Karam and Dr. Issam Kassab. The webinars' participants were based on the selection of healthcare professionals who work in both public and private sectors.
Based on the organization, proceedings, outcome, and participants’ evaluation, it is judged that the webinars were a success.
The organizers wish to acknowledge WHO represented by Dr. Omar Al Rifai for its support, the PV team for its dedication, and the participants for their willingness and input.

Awareness Campaign for COVID-19 Vaccine

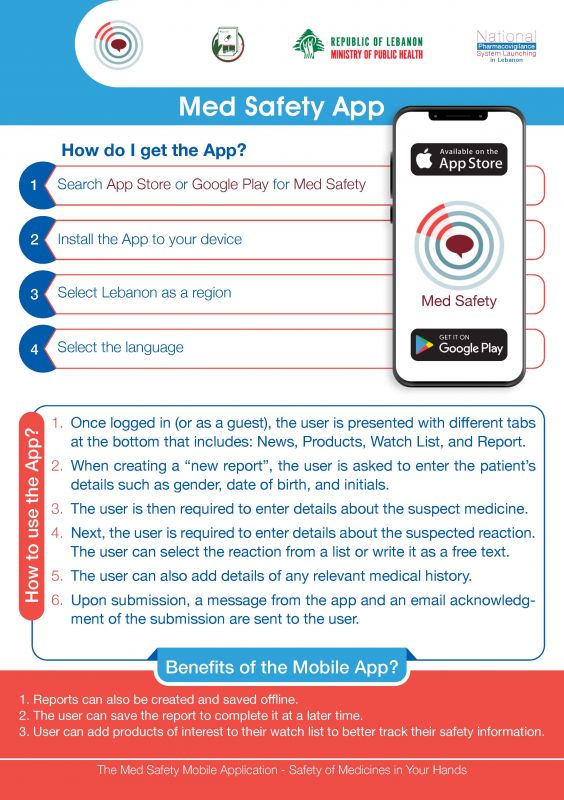
How to report adverse events folowing immunization by COVID-19 Vaccines
Pharmacovigilance Publications

Descriptive Analysis of the National Drug Adverse Events (AEs) Database in Lebanon- 2025

Serious Adverse Events Following Immunization with COVID - 19 Vaccines in Lebanon - A Retrospective Analysis of the National Pharmacoviglance Database- 2024

Trust in Pharmaceuticals and Vaccine Hesitancy - Exploring Factors Influencing COVID - 19 Immunization among Lebanese Children Aged 1 to 11 Years- 2023

A One Year Analysis of Adverse Events Following COVID - 19 Vaccination in Lebanon - A Retrospective Study- 2023
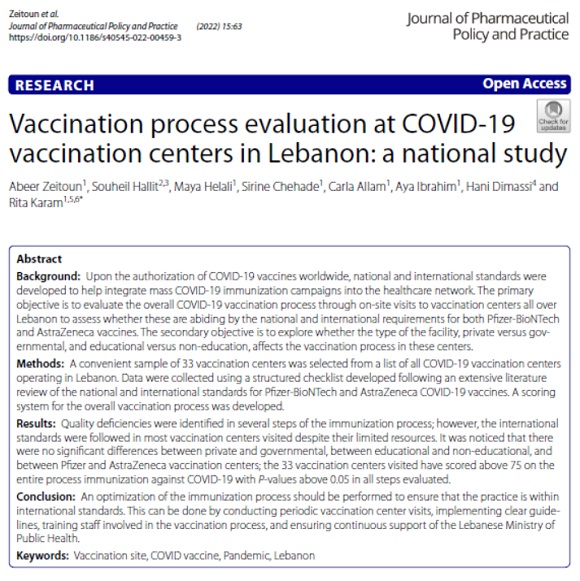
Vaccination Process Evaluation at COVID - 19 Vacination Centers in Lebanon - A National Study- 2022
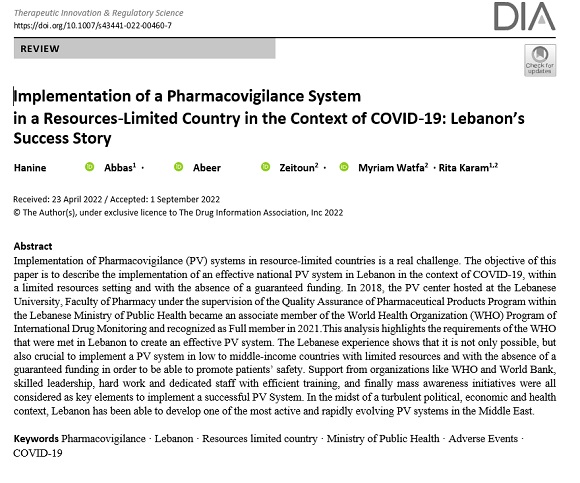
Implementation of a Pharmacovigilance System in a Resources- Limited Country in the Context of COVID - 19:Lebanon's Success Story- 2022
MedSafety Week
Briefing on the Lebanese National Pharmacovigilance Program's Participation in the Med Safety Week 2025
World Patient Safety Day (WPSD)
World Patient Safety Day 2025- 17 September 2025
PharmacoVigilance in the context of COVID-19
The current COVID-19 pandemic presents unique challenges for pharmacovigilance. According to the CIOMS/WHO Working Group, Vaccine Pharmacovigilance is the science and activities relating to the: detection, assessment, understanding, communication of Adverse Event Following Immunization (AEFIs) and other Vaccine- or Immunization-related issues, and prevention of untoward effects of the Vaccine or Immunization
According to WHO, an Adverse Event Following Immunization (AEFI) is any untoward medical occurrence which follows immunization, and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavorable or unintended sign, abnormal laboratory finding, symptom or disease.
The PharmacoVigilance Program at the MoPH is currently in charge of handling and processing all reported AEFI with the COVID-19 vaccines
According to WHO, an Adverse Event Following Immunization (AEFI) is any untoward medical occurrence which follows immunization, and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavorable or unintended sign, abnormal laboratory finding, symptom or disease.
The PharmacoVigilance Program at the MoPH is currently in charge of handling and processing all reported AEFI with the COVID-19 vaccines
Pharmacovigilance Alerts
- Medical Product Alert N°7/2025 Falsified IBRANCE (palbociclib) identified in the WHO African, Eastern Mediterranean and European regions- 15 December 2025
- Medical Product Alert N°6/2025 Falsified SIMULECT (basiliximab) for injection identified in the WHO African and European Regions- 9 December 2025
- Medical Product Alert N°5/2025 Substandard (contaminated) oral liquid medicines identified in the WHO South-East Asia Region-13 October 2025
- Medical Product Alert N°4/2025 Substandard (contaminated) FENTANILO HLB (fentanyl citrate) identified in the WHO Region of the Americas- 29 August 2025
- Medical Product Alert N°3/2025 Falsified IMFINZI (durvalumab) injection 500mg/10ml identified in the WHO Eastern Mediterranean and European Regions- May 2025
- Medical Product Alert N°2/2025 Falsified HEALMOXY (Amoxicillin) Capsules 500mg identified in the WHO African Region- April 2025
- Medical Product Alert No.1/2025- Falsified (contaminated) OXYCONTIN 80mg dentified in the WHO European Region
- Medical Product Alert No 3/2024- Falsified Contaminated Oxymorphone Hydrochloride 40mg
- Medical Product Alert No 2/2024- Falsified OZEMPIC Semaglutide
- Medical Product Alert No.1/2024- Falsified (contaminated) USPEP PROPYLENE GLYCOL
- Medical Product Alert No 8/2023 - Substandard (contaminated) syrup and suspension medicines identified in the WHO Regions of the Americas Eastern Mediterranean South-East Asia and Western Pacific
- Medical Product Alert No 7/2023 - Falsified DEFITELIO (defibrotide) identified in the WHO Regions of Europe and South-East Asia
- Medical Product Alert No 6/2023 - Substandard (contaminated) syrup medicines identified in WHO Region of the Eastern Mediterranean
- Medical Product Alert No 5/2023 - Substandard (contaminated) syrup medicines identified in WHO Region of Africa
- Medical Product Alert No 4/2023 - Substandard (contaminated) syrup medicines identified in WHO Region of the Western Pacific
- Medical Product Alert No 3/2023 - Falsified DEFITELIO (defibrotide sodium) identified in the WHO Regions of Europe and the Eastern Mediterranean
- Medical Product Alert No 2/2023- TETRACYCLINE HYDROCHLORIDE OPHTHALMIC OINTMENT USP 1%Manufactured by Galentic Pharma (India) Pvt. Ltd
- Medical Product Alert No 1/2023- Substandard (contaminated) liquid dosage medicines identified in Region
- Medical Product Alert No 8/2022 - Substandard (contaminated) METHOTREXTM 50mg identified in the WHO Eastern Mediterranean region
Reports and Executive summaries in the Context of COVID-19
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- 14th December 2022
- The Executive Summary of the 12 report
- Report 12 Supplement: Adverse Events of Special Interest
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- June 30th, 2022
- The Executive Summary of the 11th report
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- April 15th 2022
- The Executive Summary of the 10th report
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- February 19th 2022
- The Executive Summary of the 9th report
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- January 19th 2022
- The Executive Summary of the 8th report
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- November 19th 2021
- The Executive Summary of the 7th report
- Adverse Events Following Immunization Monitoring-COVID-19 Vaccines-Lebanon- 14 February 2021- October 19th 2021
- The Executive Summary of the 6th report
- Adverse Events Following Immunization Monitoring-Covid-19 Vaccines-Lebanon- 14 February 2021-September 19th, 2021
- The Executive Summary of the 5th report
- Adverse Events Following Immunization Monitoring-Covid-19 Vaccines-Lebanon- 14 February 2021-August 1st 2021
- The Executive Summary of the 4th report
- Adverse Events Following Immunization Monitoring-Covid-19 Vaccines-Lebanon- 14 February 2021-30 May 2021
- The Executive Summary of the 3rd report
- Adverse Events Following Immunization Monitoring-Covid-19 Vaccines-Lebanon- 14 February 2021-30 April 2021
- The Executive Summary of the 2nd report
- Adverse Events Following Immunization Monitoring-Covid-19 Vaccines-Lebanon- 14 February 2021-31 March 2021
- The Executive Summary of the 1st report
Reports and Executive summaries in the Context of Oral Cholera Vaccines
- Adverse Events Following Immunization Monitoring-Oral Cholera Vaccines-Lebanon- 12th November 2022- 22nd February 19th 2023 – Phase I – II - III
- The Executive Summary of the 3rd report
- Adverse Events Following Immunization Monitoring-Oral Cholera Vaccines-Lebanon- 12th November 2022- 22nd January 2023 – Phase I - II
- The Executive Summary of the 2nd report
- Adverse Events Following Immunization Monitoring-Oral Cholera Vaccines-Lebanon- 12th November 2022- 7h December 2022 – Phase I
- The Executive Summary of the 1st report
Public Awareness





Sitemap 

© Copyrights reserved to Ministry of Public Health 2026






