Are you a new member? Sign up now
Login area
| Sign up
National Tuberculosis Program
About the National Tuberculosis Program-Lebanon
NTP Centers and Services
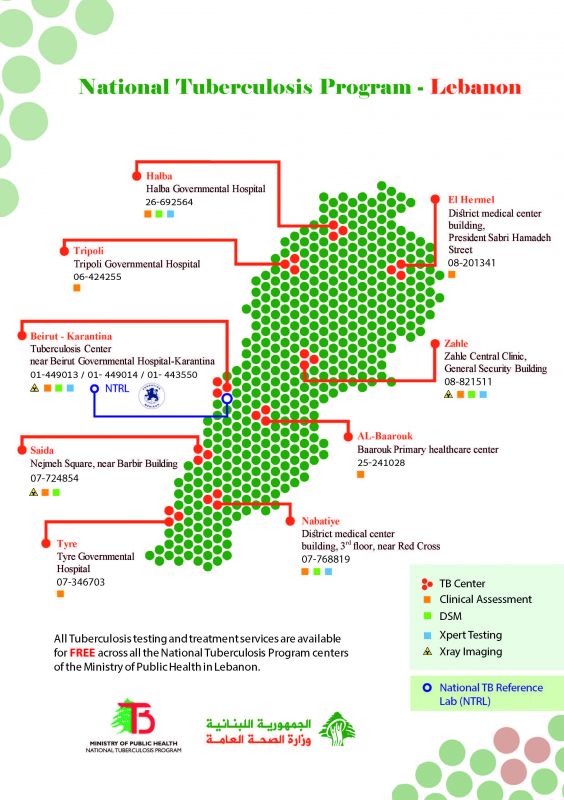
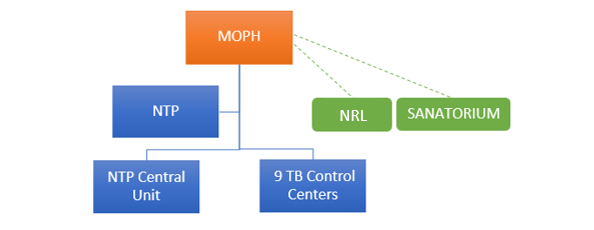
The National Tuberculosis Program (NTP), under the Ministry of Public Health of Lebanon, operates through the Tuberculosis (TB) central unit at Karantina and 9 TB control centers across the country:
1 center located in Beirut (Karantina), 1 in Mount Lebanon (Barouk), 2 in Bekaa (Zahleh and Hermel), 2 in the North (Tripoli and Halba), and 3 in the South (Nabatieh, Saida and Tyre).
NTP is committed to providing equitable, quality services for the diagnosis and treatment of TB to all notified cases residing in Lebanon, regardless of the geographical region, economic status and country of origin.
The following services are provided FREE of charge at the TB centers falling under the NTP:
- TB diagnostics and laboratory services (Chest Radiography, Direct Smear Microscopy, Gene Xpert testing, Culture and Drug Sensibility testing for first and second line drugs)
- TB cases management and treatment (first and second line treatment regimens provided for drug-susceptible and drug-resistant TB cases)
- Treatment follow-up and Support through Directly Observed Treatment (DOT) and Video Observed Treatment (VOT)
- TB Contacts investigations and Screening
- TB Prevention through the provision of preventive treatment for Latent TB cases in high risk groups when recommended (TB-HIV patients, TB Contacts, Immunocompromised patients, Migrants from high TB burden countries)
Furthermore, MOPH contracted the Sanatorium at Azounieh-Mount Lebanon for TB cases that require hospitalization and isolation.
MOPH also nominated and contracted Laboratoire Rdolphe Merieux (LRM) as the National Reference Laboratory (NRL) for TB in Lebanon as of June 2018. The following diagnostic tests are performed by the NRL as per the national TB guidelines:
MOPH also nominated and contracted Laboratoire Rdolphe Merieux (LRM) as the National Reference Laboratory (NRL) for TB in Lebanon as of June 2018. The following diagnostic tests are performed by the NRL as per the national TB guidelines:
- Culture
- Phenotypic and Genotypic Drug Susceptibility Testing for first and second line drugs
- Gene Xpert for extra-pulmonary specimens
NTP-Lebanon Organigram
TB Surveillance
TB Epidemiology in Lebanon
Lebanon is a low TB burden country with an estimated total TB incidence of 13 per 100000 populations, an estimated HIV-negative TB mortality of 1.4 per 100000 populations and a treatment coverage of 76% (WHO Global Tuberculosis Report 2021).
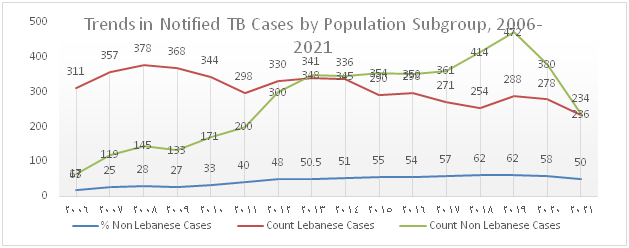
The trend of TB notification which increased from 2012 onwards due to the influx of Syrian refugees and to the migrant workforce present in the country drastically dropped over the past 2 years and this is mainly attributed to the decline in notification among migrants (Figure 1). Figure 2 shows that the percentage of foreign born TB cases which gradually increased between 2006 and 2020 dropped to 50% in 2021.
Figure 1): Trends in notified TB cases by Population Subgroups, 2007-2021
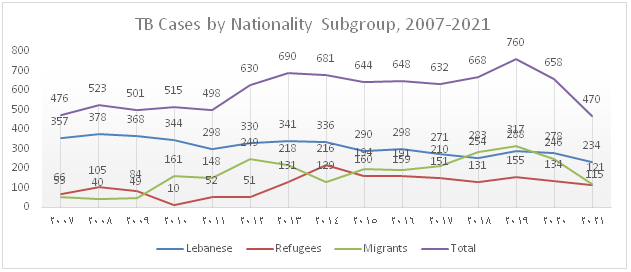
Figure 2): Trends in notified TB cases-Nationals vs. Non-nationals, 2006-2021
In 2021, 474 TB cases, including 470 DS-TB and 4 DR-TB were notified, diagnosed and enrolled under TB treatment. The total notification dropped by 38% and 28% compared to 2019 and 2020 respectively. Furthermore, the distribution by population subgroup shows a decline in notification among the different groups, particularly in migrants with a huge drop of 62% compared to 2019 and 51% compared to 2020.
A. Drug-Susceptible TB Cases
Figure 3: Distribution of TB Cases by Type of Disease

Figure 4: Distribution of EPTB Cases by Site of Disease
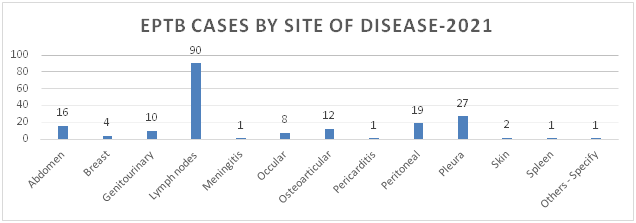
Figure 5: Distribution of TB Cases by Gender and Age Category
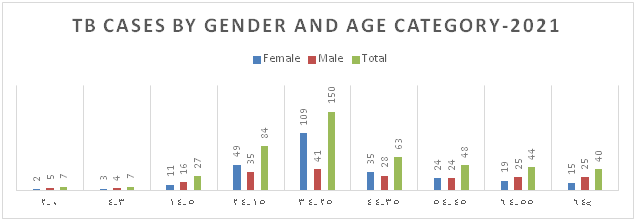
Figure 6: Distribution of TB Cases by Nationality
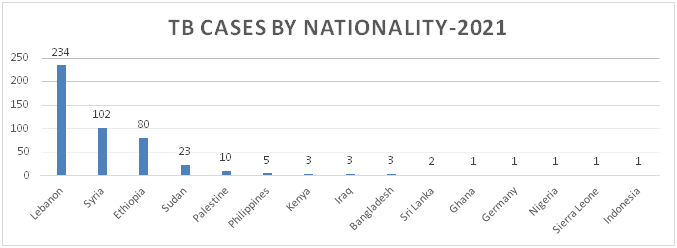
Figure 7: Distribution of TB Cases by Governorate

Figure 8.a: Distribution of TB cases by Nationality-Beirut
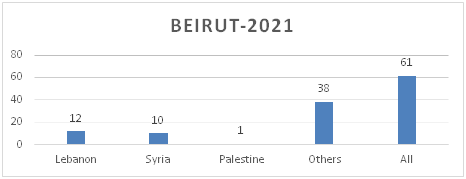
Figure 8.b: Distribution of TB cases by Nationality at District Level-Mount Lebanon
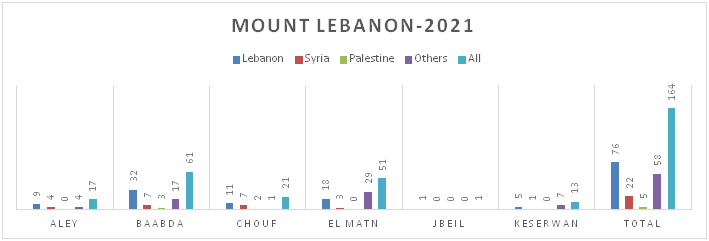
Figure 8.c: Distribution of TB cases by Nationality at District Level-North Lebanon
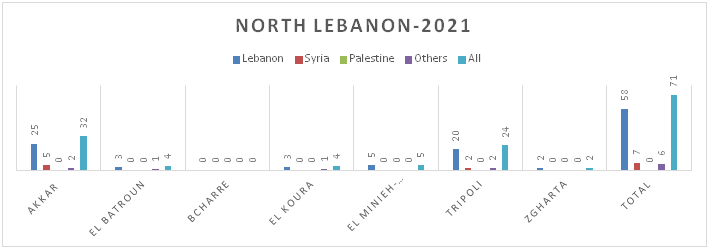
Figure 8.d: Distribution of TB cases by Nationality at District Level-South Lebanon

Figure 8.e: Distribution of TB cases by Nationality at District Level-Bekaa

B. Drug Resistant-TB Cases-2020
In total 4 cases were reported in 2021 including 2 RR-TB, 1 pre-XDR-TB and 1 XDR-TB case. 3 had PTB and 1 had EPTB; all were non-nationals.
Overall, there were 0.6% DR-TB among new notified TB cases and 17% among previously treated TB cases
Lebanon is a low TB burden country with an estimated total TB incidence of 13 per 100000 populations, an estimated HIV-negative TB mortality of 1.4 per 100000 populations and a treatment coverage of 76% (WHO Global Tuberculosis Report 2021).
The trend of TB notification which increased from 2012 onwards due to the influx of Syrian refugees and to the migrant workforce present in the country drastically dropped over the past 2 years and this is mainly attributed to the decline in notification among migrants (Figure 1). Figure 2 shows that the percentage of foreign born TB cases which gradually increased between 2006 and 2020 dropped to 50% in 2021.
Figure 1): Trends in notified TB cases by Population Subgroups, 2007-2021

Figure 2): Trends in notified TB cases-Nationals vs. Non-nationals, 2006-2021

In 2021, 474 TB cases, including 470 DS-TB and 4 DR-TB were notified, diagnosed and enrolled under TB treatment. The total notification dropped by 38% and 28% compared to 2019 and 2020 respectively. Furthermore, the distribution by population subgroup shows a decline in notification among the different groups, particularly in migrants with a huge drop of 62% compared to 2019 and 51% compared to 2020.
A. Drug-Susceptible TB Cases
Figure 3: Distribution of TB Cases by Type of Disease

Figure 4: Distribution of EPTB Cases by Site of Disease

Figure 5: Distribution of TB Cases by Gender and Age Category

Figure 6: Distribution of TB Cases by Nationality

Figure 7: Distribution of TB Cases by Governorate

Figure 8.a: Distribution of TB cases by Nationality-Beirut

Figure 8.b: Distribution of TB cases by Nationality at District Level-Mount Lebanon

Figure 8.c: Distribution of TB cases by Nationality at District Level-North Lebanon

Figure 8.d: Distribution of TB cases by Nationality at District Level-South Lebanon

Figure 8.e: Distribution of TB cases by Nationality at District Level-Bekaa

B. Drug Resistant-TB Cases-2020
In total 4 cases were reported in 2021 including 2 RR-TB, 1 pre-XDR-TB and 1 XDR-TB case. 3 had PTB and 1 had EPTB; all were non-nationals.
Overall, there were 0.6% DR-TB among new notified TB cases and 17% among previously treated TB cases
For the Public
What is Tuberculosis?
Tuberculosis (TB) is caused by bacteria (Mycobacterium tuberculosis) that most often affects the lungs and is called Pulmonary TB. It can also affect other organs in the body (such as the bones, lymph nodes, brain…) and is known as Extra-pulmonary TB.
How does it spread?
TB is spread from person to person through the air; a patient with active pulmonary TB releases the germs into the air through coughing, sneezing or spitting; a person needs to inhale only a few of these germs to become infected (Latent TB infection).
A patient with Latent TB means he has been infected by TB bacteria but has not (yet) developed active TB disease and cannot transmit it.
People infected with TB bacteria have a 5 to 15% lifetime risk of falling ill with active TB. However, persons with compromised immune systems, such as people living with HIV, malnutrition or diabetes, or people who use tobacco, have a higher risk of falling ill.
Rarely, a person could get infected with TB after the consumption of unpasteurized milk from infected cows.
What are the common signs and symptoms of TB
TB bacteria most commonly affects the lungs, and can cause the following symptoms:
Tuberculosis (TB) is caused by bacteria (Mycobacterium tuberculosis) that most often affects the lungs and is called Pulmonary TB. It can also affect other organs in the body (such as the bones, lymph nodes, brain…) and is known as Extra-pulmonary TB.
How does it spread?
TB is spread from person to person through the air; a patient with active pulmonary TB releases the germs into the air through coughing, sneezing or spitting; a person needs to inhale only a few of these germs to become infected (Latent TB infection).
A patient with Latent TB means he has been infected by TB bacteria but has not (yet) developed active TB disease and cannot transmit it.
People infected with TB bacteria have a 5 to 15% lifetime risk of falling ill with active TB. However, persons with compromised immune systems, such as people living with HIV, malnutrition or diabetes, or people who use tobacco, have a higher risk of falling ill.
Rarely, a person could get infected with TB after the consumption of unpasteurized milk from infected cows.
What are the common signs and symptoms of TB
TB bacteria most commonly affects the lungs, and can cause the following symptoms:
- Cough that lasts 2 weeks or longer
- Chest pain
- Fatigue
- Loss of appetite
- Weight loss
- Fever
- Night Sweats
How is it treated?
Active, drug-susceptible TB disease is treated with a standard 6 months course of 4 antimicrobial drugs that are provided with information and support to the patient. TB disease can be cured when medicines are taken properly. You should inform your doctor of any adverse event that might occur throughout the treatment course.
Drug resistance emerges when anti-TB medicines are used inappropriately (such as when a patient stops treatment prematurely) and can lead to the development of a dangerous, life threatening form of TB disease.
DOT-VOT
Directly Observed Therapy (DOT) and Video Observed Treatment (VOT) improve treatment adherence by requiring a health worker or volunteer to observe patients taking each dose of the medicine on a daily basis.
TB is preventable and curable. Keep in mind that compliance to treatment is critical for cure and always follow the instructions of your healthcare provider.
How to prevent the spread of TB?
If you have tuberculosis, you should follow a number of preventive measures that help in reducing the transmission and spread of the disease especially to household members and close people.
- Do not spit on the ground
- Do not cough or sneeze in front of others without covering your mouth or nose
- Maintain good ventilation of the house
- Allow natural sunlight into the house
For Professionals
Lebanon National Strategic Plan to End Tuberculosis, 2023-2030
National TB Guidelines (Links)
- National Guidelines for Drug Susceptible Tuberculosis Lebanon- 2023
- Drug Resistant TB Guidelines -2021
- National TB Guidelines 2017-English
- National TB Guidelines 2017-French
TB Algorithms (Links)
- Algorithm for the Diagnosis of TB- 2021
- Algorithm for the Diagnosis of Drug Resistant TB- 2021
- Algorithm for Latent TB-English
- Algorithm for Latent TB-French
- Algorithm for Active Pulmonary TB-English
- Algorithm for Active Pulmonary TB-French
- Algorithm for Drug Resistant TB-English
- Algorithm for Drug Resistant TB-French
Annual TB Reports (Links)
- NTP-NAP Annual Report – 2024
- NTP Annual Report – 2023
- NTP Annual Report – 2022
- NTP Annual Report – 2021
- NTP Annual Report – 2020
- NTP Annual Report – 2019
IEC Materials
Sputum collection
Coughing Etiquette
TB Symptoms-1
TB Symptoms-2
TB Symptoms-3
General facts about TB-Ar
كيفية جمع عينة البلغم
آداب السعال
عوارض السل-1
عوارض السل-2
عوارض السل-3

















Coughing Etiquette
TB Symptoms-1
TB Symptoms-2
TB Symptoms-3
General facts about TB-Ar
كيفية جمع عينة البلغم
آداب السعال
عوارض السل-1
عوارض السل-2
عوارض السل-3

















Sitemap 

© Copyrights reserved to Ministry of Public Health 2025